Origin of life: Transitioning to DNA genomes in an RNA world
For as long as history has been recorded, humanity has tried to answer the ancient question of our origins. The ‘central dogma’ of molecular biology, first stated by Francis Crick in 1958, represented a major step forward in our efforts to answer this question (Figure 1A; Crick, 1958). In this model, the genetic information stored in DNA is transcribed to produce RNA, which is then translated by the ribosome to produce chains of amino acids. These chains fold to make the proteins that are responsible for almost everything that happens in cells.
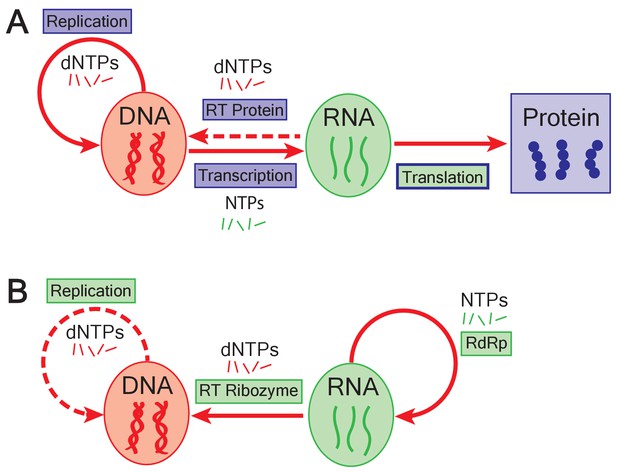
The emergence of DNA genomes in the RNA world.
(A) In the central dogma of molecular biology, information flows from DNA (red oval) to RNA (green oval) to protein (blue box). DNA is formed of building blocks called deoxynucleoside triphosphates (dNTPs) and can be replicated (solid looping red arrow); RNA is formed of nucleoside triphosphates (NTPs). Enzymes called reverse transcriptases (RT) enable complementary DNA to be made from the building blocks of RNA (dashed arrow). Blue rectangles represent processes catalyzed by proteins; green rectangles show processes catalyzed by RNA; translation is mediated by an RNA catalyst (green inner rectangle) that has proteins that modulate its activity (blue outline). (B) In the RNA world, ribozymes (RdRp) replicate RNA genomes (solid looping red arrow). Based on the work of Joyce and Samanta, if dNTPs were present in the RNA world, reverse transcriptase ribozymes could have constructed DNA genomes using RNA genomes as a template (straight red arrow). Ribozymes could also have potentially replicated DNA genomes (dashed red arrow).
The flow of information from DNA to RNA to protein is thought to have evolved out of a simpler evolutionary period when genetic information was stored and transmitted solely by RNA molecules. This theory, known as the ‘RNA world hypothesis’, posits that an RNA enzyme or ‘ribozyme’ capable of copying RNA molecules existed early in evolution, and that protein synthesis by the ribosome (which is also an RNA enzyme) evolved out of this system (Figure 1B; Gilbert, 1986; Atkins et al., 2011). The theory, however, is largely silent on how DNA genomes evolved.
In modern metabolism, protein-based enzymes called reverse transcriptases can copy RNA to produce molecules of complementary DNA. Other enzymes can promote the production of DNA nucleotides (the building blocks of DNA molecules) from RNA nucleotides via challenging chemical reactions. So how did the first DNA genomes come to be? There are two possibilities within the framework of the RNA world. In the first, protein enzymes evolved before DNA genomes. In the second, the RNA world contained RNA polymerase ribozymes that were able to produce single-stranded complementary DNA and then convert it into stable double-stranded DNA genomes.
A number of laboratories around the world are trying to build ribozymes that can sustain RNA replication (Wang et al., 2011; Attwater et al., 2013). Recently, David Horning and Gerald Joyce artificially evolved a ribozyme that is capable of copying complex RNAs and amplifying short RNA templates (Horning and Joyce, 2016). Now, in eLife, Joyce and Biswajit Samanta at the Salk Institute demonstrate that this ribozyme is also a reverse transcriptase (Samanta and Joyce, 2017). Feeding DNA nucleotides to this ribozyme enabled it to copy short segments of RNA templates into complementary DNA. This suggests that if an RNA world contained DNA nucleotides, DNA genomes could have been assembled and then presumably replicated by ribozymes.
Whether DNA genomes existed very early in evolution fundamentally rests on whether DNA nucleotides were available in the RNA world. There are plausible routes by which RNA and DNA nucleotides could have been synthesized before life emerged, meaning that they are likely to have been available at the dawn of an RNA world (Ritson and Sutherland, 2014; Becker et al., 2016; Kim and Benner, 2017). Likewise, artificially selected ribozymes have been used to synthesize the two types of bases found in RNA nucleotides from simpler precursors, suggesting RNA nucleotides could have been built by early RNA systems (Martin et al., 2015). If DNA precursors were also available early in evolution, then the synthesis of DNA nucleotides by an RNA system appears likely. While this area is currently underexplored experimentally, there appears to be no fundamental reason why DNA nucleotides could not have been abundant quite early in evolution.
Demonstrating that DNA polymerase ribozymes are able to rapidly use such DNA nucleotides would represent a major step forward for the early DNA genome model. While the field of artificial RNA polymerase ribozymes has made rapid strides, their ability to add multiple nucleotides rapidly is still very limited. Current ribozymes are significantly longer and more complex than the sequences that they are able to copy, but to make self-evolving systems, ribozymes need to be able to copy sequences that are longer and more complex than themselves. It will therefore be exciting to see if the techniques that have created such RNA polymerases are also able to evolve DNA polymerase ribozymes that have the potential to make self-replicating systems using DNA and not RNA as a source of genetic material. Such a system would bring us closer to understanding the transition from an RNA world to a type of life that respects the rules of the central dogma of modern biology.
References
-
In-ice evolution of RNA polymerase ribozyme activityNature Chemistry 5:1011–1018.https://doi.org/10.1038/nchem.1781
-
On protein synthesisSymposia of the Society for Experimental Biology 12:138–163.
-
Conversion of biosynthetic precursors of RNA to those of DNA by photoredox chemistryJournal of Molecular Evolution 78:245–250.https://doi.org/10.1007/s00239-014-9617-0
Article and author information
Author details
Publication history
- Version of Record published: November 1, 2017 (version 1)
Copyright
© 2017, Cojocaru et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 16,623
- views
-
- 792
- downloads
-
- 6
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Biochemistry and Chemical Biology
- Evolutionary Biology
Stramenopiles form a clade of diverse eukaryotic organisms, including multicellular algae, the fish and plant pathogenic oomycetes, such as the potato blight Phytophthora, and the human intestinal protozoan Blastocystis. In most eukaryotes, glycolysis is a strictly cytosolic metabolic pathway that converts glucose to pyruvate, resulting in the production of NADH and ATP (Adenosine triphosphate). In contrast, stramenopiles have a branched glycolysis in which the enzymes of the pay-off phase are located in both the cytosol and the mitochondrial matrix. Here, we identify a mitochondrial carrier in Blastocystis that can transport glycolytic intermediates, such as dihydroxyacetone phosphate and glyceraldehyde-3-phosphate, across the mitochondrial inner membrane, linking the cytosolic and mitochondrial branches of glycolysis. Comparative analyses with the phylogenetically related human mitochondrial oxoglutarate carrier (SLC25A11) and dicarboxylate carrier (SLC25A10) show that the glycolytic intermediate carrier has lost its ability to transport the canonical substrates malate and oxoglutarate. Blastocystis lacks several key components of oxidative phosphorylation required for the generation of mitochondrial ATP, such as complexes III and IV, ATP synthase, and ADP/ATP carriers. The presence of the glycolytic pay-off phase in the mitochondrial matrix generates ATP, which powers energy-requiring processes, such as macromolecular synthesis, as well as NADH, used by mitochondrial complex I to generate a proton motive force to drive the import of proteins and molecules. Given its unique substrate specificity and central role in carbon and energy metabolism, the carrier for glycolytic intermediates identified here represents a specific drug and pesticide target against stramenopile pathogens, which are of great economic importance.
-
- Biochemistry and Chemical Biology
Protein phosphorylation is one of the major molecular mechanisms regulating protein activity and function throughout the cell. Pannexin 1 (PANX1) is a large-pore channel permeable to ATP and other cellular metabolites. Its tyrosine phosphorylation and subsequent activation have been found to play critical roles in diverse cellular conditions, including neuronal cell death, acute inflammation, and smooth muscle contraction. Specifically, the non-receptor kinase Src has been reported to phosphorylate Tyr198 and Tyr308 of mouse PANX1 (equivalent to Tyr199 and Tyr309 of human PANX1), resulting in channel opening and ATP release. Although the Src-dependent PANX1 activation mechanism has been widely discussed in the literature, independent validation of the tyrosine phosphorylation of PANX1 has been lacking. Here, we show that commercially available antibodies against the two phosphorylation sites mentioned above—which were used to identify endogenous PANX1 phosphorylation at these two sites—are nonspecific and should not be used to interpret results related to PANX1 phosphorylation. We further provide evidence that neither tyrosine residue is a major phosphorylation site for Src kinase in heterologous expression systems. We call on the field to re-examine the existing paradigm of tyrosine phosphorylation-dependent activation of the PANX1 channel.






