Abstract
Selective serotonin reuptake inhibitors (SSRIs) for treatment of prenatal maternal depression have been associated with neonatal neurobehavioral disturbances, though the molecular mechanisms remain poorly understood. In utero exposure to SSRIs may affect DNA methylation (DNAme) in the human placenta, an epigenetic mark that is established during development and is associated with gene expression. Chorionic villus samples from 64 human placentas were profiled with the Illumina MethylationEPIC BeadChip; clinical assessments of maternal mood and SSRI treatment records were collected at multiple time points during pregnancy. Case distribution was 20 SSRI-exposed cases and 44 SSRI non-exposed cases. Maternal depression was defined using a mean maternal Hamilton Depression score > 8 to indicate symptomatic depressed mood (“maternally-depressed”), and we further classified cases into SSRI-exposed, maternally-depressed (n = 14); SSRI-exposed, not maternally-depressed (n = 6); SSRI non-exposed, maternally-depressed (n = 20); and SSRI non-exposed, not maternally-depressed (n = 24). For replication, Illumina 450K DNAme profiles were obtained from 34 additional cases from an independent cohort (n = 17 SSRI-exposed, n = 17 SSRI non-exposed). No CpGs were differentially methylated at FDR < 0.05 comparing SSRI-exposed to non-exposed placentas, in a model adjusted for mean maternal Hamilton Depression score, or in a model restricted to maternally-depressed cases with and without SSRI exposure. However, at a relaxed threshold of FDR < 0.25, five CpGs were differentially methylated (|Δβ| > 0.03) by SSRI exposure status. Four were covered by the replication cohort measured by the 450K array, but none replicated. No CpGs were differentially methylated (FDR < 0.25) comparing maternally depressed to not depressed cases. In sex-stratified analyses for SSRI-exposed versus non-exposed cases (females n = 31; males n = 33), three additional CpGs in females, but none in males, were differentially methylated at the relaxed FDR < 0.25 cut-off. We did not observe large-scale alterations of DNAme in placentas exposed to maternal SSRI treatment, as compared to placentas with no SSRI exposure. We also found no evidence for altered DNAme in maternal depression-exposed versus depression non-exposed placentas. This novel work in a prospectively-recruited cohort with clinician-ascertained SSRI exposure and mood assessments would benefit from future replication.
Similar content being viewed by others
Introduction
As many as 20% of pregnant individuals are affected by mood disorders such as depression during pregnancy, and up to 6% of all pregnant individuals are treated with antidepressants such as selective serotonin reuptake inhibitors (SSRIs), based on estimates from several North American and European studies1,2,3,4. Clinical management decisions for treatment of perinatal depression are complex, and in each case the clinician must balance the risks of treatment with the severity of depressive symptoms5. Given the prevalence of maternal depression and SSRI treatment during gestation, much research has been conducted focusing on the fetal effects of both6,7.
Maternal prenatal depressed mood has been associated with preterm birth (< 37 weeks of gestation), growth and developmental delays, and increased postnatal infant stress8,9. Additionally, the literature suggests that the presence of maternal depressive symptoms independent of SSRI therapy is associated with increases in offspring childhood internalizing behaviours and affective disorders, though these exposures are hard to separate in human studies10. Animal studies have permitted further investigation into the effect of maternal depressed mood on offspring outcomes by enabling the randomization of SSRI treatment. Rodent models have confirmed that associations identified between maternal depressed mood and offspring anxiety disorders are not mediated by SSRI exposure, and that SSRI treatment does not protect offspring from these outcomes; this topic is reviewed in10.
Regarding the impacts of SSRI treatment on the feto-placental unit, Oberlander et al. previously found that maternal SSRI treatment was associated with altered blood flow to the fetus, increased rates of fetal hypoxemia, altered early neurodevelopment, lower arousal index throughout the newborn period, and altered psychomotor test scores6,7,11,12,13. Prenatal SSRI exposure also tended to be associated with lower fetal-placental weight ratios and poorer fetal vascular perfusion, indicative of less efficient fetal blood flow to or from the placenta14. Both of these factors indicate less efficient placental function in association with prenatal SSRI exposure, which may be related to downstream neonatal cognitive outcomes14. Exposure to maternal SSRI treatment has previously been reported to affect syncytializaton of placental trophoblasts and extravillous trophoblast function, and to affect placental serotonin levels15,16,17. Taken together, the effect of SSRI exposure on pregnancy outcomes suggests an interplay between the placenta and SSRI pharmacobiology, which could occur by a variety of mechanisms. It has, however, been postulated that both depression and SSRI exposure may impact fetal outcomes via altered serotonin signalling and downstream effects.
Serotonin (5-hydroxytryptamine, or 5-HT) is a central neurotransmitter and a critical signaling factor in many contexts, including during prenatal neurodevelopment of the fetus18. Low levels of serotonin in the central nervous system have historically been associated with depressive symptoms in adults, in the 5-HT theory of depression19. SSRI therapeutics, which increase extracellular levels of 5-HT, have been associated with alleviation of depressive symptoms20. In vivo, serotonin is synthesized from the essential amino acid l-tryptophan, by tryptophan hydroxylase enzymes 1 and 2 (TPH-1, TPH-2), and is degraded by monoamine oxidases A and B (MAOA, MAOB)21,22. SSRIs function to block the serotonin transporter (SERT, also known as 5-HTT (5-hydroxytryptamine transporter)), inhibiting the reabsorption of 5-HT by the presynaptic neurons and thereby increasing extracellular serotonin levels in the central nervous system13. Several aspects of serotonin and SSRI biology are relevant to prenatal development, and both maternal depression and SSRI exposure have been associated with altered fetal outcomes. Notably, serotonin is first expressed early in gestation, when placental TPH-1 and TPH-2 convert maternal l-tryptophan to serotonin de novo22,23. Over the course of gestation, serotonin (5-HT) is essential to many processes of prenatal development including embryogenesis, placentation, and neurodevelopment21,24,25. SSRIs readily cross the placenta, leading to fetal drug exposure and altered fetal cardiac autonomic activity, presumably via altered serotonin signaling in the placenta and/or fetus26,27.
One plausible mechanism by which SSRI exposure may impact pregnancy outcomes is via DNA methylation (DNAme). DNAme is an epigenetic mark involving the addition of a methyl group to the 5′ carbon of a cytosine molecule, which usually occurs in the context of cytosine-guanine dinucleotides (CpGs). In human tissues, DNAme signatures are associated with gene expression patterns28, and are altered in association with certain environmental and chemical exposures, such as air pollution, arsenic, and tobacco smoke; for a review of this topic see29.
DNAme patterns in the human placenta vary in association with certain maternal exposures and environments, but are relatively stable once established, and serve as a possible record of gene expression patterns and exposures during gestation30,31. The placenta exists at the interface of the maternal environment and the developing fetus, and environmental factors can gain access to the fetus in many cases only via the placenta32,33. As SSRIs readily cross the placenta34, it is possible that SSRI species alter placental DNAme patterns directly via association with epigenetic readers and writers (DNMT or TET enzymes), or through altering gene expression patterns in the serotonin signalling pathway, or other related pathways29.
In this study, we hypothesized that prenatal SSRI treatment would be associated with distinct placental DNAme signatures. In a prospective cohort of 64 placentas with extensive maternal mood assessments during gestation, both with and without SSRI exposure, we investigated the effects of maternal SSRI treatment and maternal depressed mood on placental DNA methylation profiles. We considered the effect of SSRI exposure on placental DNAme as our primary outcome. The secondary outcome considered was the impact of maternal depression, which was investigated only in participants not treated with SSRIs. Additionally, given previously reported sex differences in neurodevelopmental and placental outcomes related to maternal stress exposure35,36,37,38,39,40, we also assessed placental DNAme associated with maternal SSRI treatment in separate sex-stratified models.
Methods
Discovery cohort
Participants were recruited as part of a prospective, longitudinal cohort approved by the University of British Columbia/Children’s and Women’s Health Centre of British Columbia research ethics board: H12-0073340. Written informed consent was obtained from all participants, and all procedures complied with the ethical standards on human experimentation and with the Helsinki Declaration of 1975 (revised in 2008). This specific study was additionally approved under certificate H16-02280.
Pregnant individuals with and without prenatal diagnoses of clinical depression, and with/without SSRI treatment plans were recruited at the British Columbia Women’s Hospital (BCWH) in the 20th week of gestation (n = 64). At recruitment, all pregnant individuals received the MINI assessment to screen for DSM-IV Axis I Depression. SSRI use was reported by the treating clinicians, and was defined as treatment for at least 75 days including the 3rd trimester with one of the following: fluoxetine (Prozac), paroxetine (Paxil), sertraline (Zoloft), citalopram (Celexa), escitalopram (Lexapro), or venlafaxine (Effexor). Compliance with SSRI treatment was assessed and recorded at each prenatal study visit. While placental drug concentrations were not available at the time of this study, previous work on this cohort indicated that maternal plasma drug concentrations during gestation were consistent with self-report of SSRI use26. Maternal mood was assessed at recruitment, 36 weeks of gestation, postnatal day 6, and at 24 months postnatally. The mood assessment included multiple clinician and patient-rated measures, of which the Hamilton Depression score, a 17-item clinician implemented assessment of the severity of depressive symptoms41, and the Edinburgh Postnatal Depression score (EPDS), a 10-item self-assessment screening tool for depression42, were analyzed.
Exclusion criteria were: bipolar illnesses, hypertension, any diabetes, current substance abuse, placental insufficiency, multi-fetal pregnancies, infants with congenital brain malformations, intrauterine growth restriction, or preterm birth. For all participants, measures of obstetric history, prenatal medication use, and sociodemographic variables were obtained. At birth, additional clinical information was collected for each infant including gestational age at delivery, infant sex assigned at birth, and infant birth weight; placentas were collected for DNAme studies.
After delivery, chorionic villus samples were obtained from 4 distinct cotyledonary sites (1.5–2 cm3) below the surface of the fetal-facing side of each placenta, as previously described43. DNA was extracted from each of the 4 sites and pooled in equimolar amounts to provide a representative sample of the whole placenta prior to obtaining DNAme profiles. DNAme data for these 64 cases was collected in a single processing batch using the Illumina MethylationEPIC (“EPIC”) array platform (Illumina, San Diego, CA, USA), which profiles > 850,000 CpGs genome-wide.
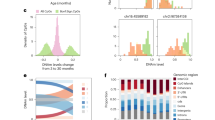
Case distribution was as follows: 20 SSRI-exposed and 44 SSRI non-exposed samples (Table 1). Cases were additionally sub-categorized into four groups for analysis based on both SSRI exposure and maternal depression status using a mean maternal Hamilton Depression score of > 8 to indicate symptomatic depressed mood (“maternally depressed”) as follows: SSRI-exposed, maternally-depressed (n = 14); SSRI-exposed without maternal depression (n = 6); SSRI non-exposed, maternally depressed (n = 20); and SSRI non-exposed without maternal depression (n = 24), see Table 2 and Fig. 1.
Comparisons for analysis of differential DNAme associated with SSRI exposure and maternal depression in the discovery cohort. (A) The SSRI model investigates SSRI exposure in the full cohort (n = 64), comparing SSRI-exposed (n = 20) to SSRI non-exposed (n = 44) cases, adjusting for mean maternal Hamilton Depression score across gestation. (B) The SSRIs in maternal depression model investigates SSRI exposure in all maternally-depressed cases (mean maternal Hamilton Depression score > 8, n = 34), this model compares SSRI-exposed, maternally depressed cases (n = 14) to SSRI non-exposed, maternally depressed cases (n = 20). (C) The maternal depression without SSRIs model investigates the effect of maternal depression in cases not exposed to SSRIs (n = 44), this model compares SSRI non-exposed, maternally depressed cases (n = 20) to SSRI non-exposed and not maternally depressed cases (n = 24).
Replication cohort
An independent cohort of 34 cases with (i) known SSRI-exposure status and (ii) pre-collected placental DNAme data were obtained from a subset of the Rhode Island Child Healthy Study (RICHS)44. Cohort participants were treated with one of the following SSRIs: fluoxetine, paroxetine (Paxil), sertraline (Zoloft), citalopram (Celexa), or escitalopram (Lexapro). Assessments of maternal mood were not available for these cases. DNAme data for these 34 cases were previously collected with the Illumina HumanMethylation450 (“450K”) array platform, and are available on the Gene Expression Omnibus (GEO) repository under accession number GSE7524844.
Data processing
Processing and normalization of the Illumina EPIC DNAme data for the discovery cohort is described in full in the Supplementary Methods. First, sample sex (XY chromosome complement) and genetic uniqueness were assessed and confirmed to match the annotated metadata. Next, polymorphic and cross-hybridizing probes were removed from this dataset47, as were probes with a detection p value > 0.01 in > 5% of cases. Subsequently, CpGs with non-variable DNAme in this cohort (n = 87,572) were removed from the dataset to decrease multiple test correction burden. Non-variable CpGs were defined as in48 as CpGs that met both of the following conditions: (i) < 5% range in DNAme beta (β) values between the 10th-90th centile, and (ii) previously reported as placenta-non-variable in49. After processing and dasen + noob normalization50 659,036 autosomal CpGs in 64 cases, plus 8 pairs of technical replicates, remained for analysis. The technical replicates were used to estimate an absolute delta beta (|Δβ|) cut-off for subsequent analyses, based on the average root mean squared error of the technical replicate pairs (0.026) and the average standard error of all CpGs in all cases (0.0048). The highest of these values was selected and rounded up, to establish a |Δβ| cut-off of > 0.03 between groups; anything less than |Δβ| = 0.03 could represent technical noise and was unlikely to be biologically meaningful in this cohort. After processing, the replicate pairs were removed from the cohort and excluded from downstream analysis.
Batch correction was performed after identifying an effect of EPIC array row on DNAme data using Principal Components Analysis, see Supplementary Fig. S1. Prior to batch correction, the distribution of cases across EPIC array rows was confirmed to be independent of our primary outcomes of interest: SSRI status (Fisher test p > 0.05) and mean maternal Hamilton depression score (ANOVA p > 0.05), see Supplementary Fig. S2. ComBat was used to correct the remaining categorical effect of the “row” variable, with SSRI exposure (yes/no) and mean maternal Hamilton Depression score (continuous) included as variables of interest in the ComBat model matrix as recommended by the sva package authors51. Results for batch-corrected analyses were confirmed in non-batch-corrected data to ensure that false signal was not introduced during batch correction, in accordance with the recommendations outlined in52, see Supplementary Fig. S1.
Replication cohort
The raw data for the full 335-sample RICHS cohort was downloaded from GEO (GSE75248) and processed analogously to the discovery cohort: first sample sex and genetic uniqueness were checked, data were dasen + noob normalized, and polymorphic and cross-hybridizing probes were removed47, as were probes with a detection p value > 0.01 in > 5% of cases. For a complete description of data processing see the Supplementary Methods. The processed data were subsetted to 34 cases for which gestational SSRI treatment status was recorded in the medical chart. Replication cohort demographics are presented in Table 1.
Covariate selection
We next sought to identify characteristics that could be associated with SSRI exposure in the discovery and replication cohorts, which should be included as covariates in statistical models. Demographic variables considered include those presented in Tables 1 and 2. The discovery cohort was well-balanced across all demographic variables by both SSRI exposure and maternal depression status. Gestational age, infant sex, and PlaNET ancestry were selected for inclusion as additive covariates in linear modelling analyses as it is known that these factors drive large amounts of DNAme variation in the placenta46,53. The same variables were selected in the replication cohort (infant sex, gestational age at birth, PlaNET ancestry), with the addition of type of delivery (vaginal/other), as delivery type frequency differed by SSRI exposure in the replication cohort only, see Table 1.
Using tools from the PlaNET R package54,55, we also estimated additional variables in the discovery cohort from the DNAme data itself: the proportion of six major placental cell types (cytotrophoblasts, syncytiotrophoblasts, endothelial cells, stromal cells, Hofbauer cells, and nucleated red blood cells), and placental epigenetic age. Cell type proportions were not significantly associated with either SSRI treatment or mean maternal Hamilton Depression score across gestation (t-test for cell type proportions ~ SSRI exposed (yes/no), all p values > 0.05; Pearson correlation for mean maternal Hamilton Depression score and each cell type proportion, all p values > 0.05), see Supplementary Fig. S3. Accordingly, cell type proportions were not included as covariates in subsequent linear models. Epigenetic age acceleration was calculated as the residual of the Control Placental Clock epigenetic age regressed on gestational age at birth, adjusted for sex, ancestry, and EPIC array row. Intrinsic epigenetic age acceleration (cell-type independent) was calculated similarly, but with additional adjustment for numeric cell type proportions. Placental epigenetic age was not considered as a possible covariate in linear models, but was analyzed separately for associations with SSRI exposure as previous studies have reported conflicting relationships between cord blood epigenetic age acceleration and SSRI treatment, so parallel exploration in the placenta was warranted56,57, see “Results”.
Linear modelling to identify placental DNAme associated with SSRI exposure and maternal depression
To identify differential DNAme associated with both SSRI exposure and exposure to maternal depression in the discovery cohort, three linear models were explored, see Fig. 1. Linear modelling on M values was conducted using limma in R58,59. In addition to the main effects, all models were adjusted for infant sex, gestational age at birth (in weeks), EPIC array row, and two of three PlaNET ancestry coordinates46. EPIC array row was included as a covariate according to the recommended implementation of ComBat per the sva package authors51. In (A) the SSRI model, the full cohort was used for a linear model to assess the effect of SSRI exposure (nexposed = 20 versus nnon-exposed = 44) on placental DNAme, adjusting for mean maternal Hamilton Depression score across gestation. In (B) the SSRIs in maternal depression model, we used only cases with maternal depression (mean maternal Hamilton Depression score > 8, n = 34) and assessed the effect of SSRI exposure in exposed (n = 14) versus SSRI non-exposed cases (n = 20). In (C) the maternal depression without SSRIs model, we used only cases without SSRI exposure during gestation (n = 44), and assessed the effect of maternal depression alone by comparing SSRI non-exposed, maternally-depressed cases (n = 20) to not maternally-depressed cases (n = 24). Multiple test correction for all models was performed using the Benjamini–Hochberg false discovery rate method60.
For replication analyses, linear models were run only on the CpGs of interest (M values), identified in the discovery cohort models A–C. Replication linear models were adjusted for infant sex, gestational age at birth, two of three PlaNET ancestry coordinates, and type of delivery (vaginal/other). CpGs were considered to replicate differential DNAme in association with SSRI exposure at a nominal p value < 0.05 in the replication cohort.
Ethics approval and consent to participate
Participants were recruited as part of a prospective, longitudinal cohort approved by the University of British Columbia Children's and Women's Health Centre of British Columbia Research Ethics Board (UBC C&W REB) (H12-0073340), this sub-study was also approved by the same ethics boards (H16-02280).
Results
SSRI exposure and maternal depression are not associated with widespread alterations in placental DNAme patterns
First, we assessed in separate models whether DNAme was altered in placentas with SSRI exposure or exposure to maternal depression during gestation. No CpGs were significantly differentially methylated at the commonly used statistical threshold of FDR < 0.05 in any of the three models tested. Plotting FDR against the |Δβ| for all CpG sites tested in the three models demonstrated very few DNAme associations with either SSRI exposure or maternal depression array-wide, see Fig. 2.
Volcano plots for differential DNAme in association with SSRI exposure and categorical depression. For all plots HamD refers to the mean maternal Hamilton Depression score across gestation for each individual. False discovery rate (FDR) is shown along the Y axis with more significant (lower FDR) values at the top of the plot. Vertical dashed intercepts demarcate |Δβ| = 0.03, horizontal dashed intercepts indicate, from top to bottom, FDR = 0.05, FDR = 0.15, and FDR = 0.25. (A) Volcano plot for the SSRI model, investigating differential DNAme associated with SSRI exposure adjusted for mean maternal HamD score, (n = 64). Difference in DNAme (Δβ) is plotted along the X axis and was calculated as Δβ = βSSRI-exposed – βSSRI non-exposed. (B) Volcano plot for the SSRIs in maternal depression model, differential DNAme associated with SSRI exposure in cases with mean maternal HamD score > 8, (n = 34). Difference in DNAme (Δβ) is plotted along the X axis and was calculated as Δβ = βSSRI-exposed – βSSRI non-exposed. (C) Volcano plot for the maternal depression without SSRIs model, differential DNAme associated HamD score > 8 across gestation, in SSRI non-exposed cases, (n = 44). Difference in DNAme (Δβ) is plotted along the X axis and was calculated as Δβ = βHamilton > 8 – βHamilton ≤ 8, “Depr” indicates “Depressed”, i.e. HamD > 8.
Since the relatively small sample size of the discovery cohort could limit our ability to detect significant between-group DNAme differences, in addition to the standard threshold of FDR < 0.05, we also evaluated CpGs that met more relaxed thresholds of FDR < 0.15 and FDR < 0.25. As these thresholds are associated with increased expected proportions of false positive findings, we have labelled FDR < 0.15 hits “moderate-confidence” and FDR < 0.25 “low-confidence”. For both FDR < 0.15 and FDR < 0.25 thresholds, we maintained a minimum effect size threshold of |Δβ| > 0.03.
At these relaxed thresholds, in the SSRI model (model A) adjusted for mean maternal Hamilton Depression score, one CpG was differentially methylated at FDR < 0.15, and one more at FDR < 0.25, see Fig. 2 and Supplementary Table S1. The one differentially methylated CpG at FDR < 0.15 (cg12900404) exhibited a Δβ of + 0.11 (higher average DNAme β value in SSRI-exposed placentas). Loosening the threshold to FDR < 0.25, one additional CpG was differentially methylated by SSRI exposure status (cg20877313), and was also more highly methylated in SSRI-exposed placentas (Δβ = + 0.04). In the SSRIs in maternal depression model (model B), three CpGs were differentially methylated at the moderate-confidence threshold of FDR < 0.15 (cg12655501, cg26993610, cg14340829). These CpGs all had an average difference in DNAme between SSRI-exposed and SSRI non-exposed cases of |Δβ| > 0.10. In the maternal depression without SSRIs model (model C), no CpGs showed evidence of maternal depression-associated differential DNAme at any FDR threshold. For a description of all CpGs that satisfied the moderate and low-confidence FDR thresholds from the SSRI models (A, B) and SSRIs in maternal depression model (C), including test statistics and overlapping genes, see Table 3. Summary statistics of linear modelling from all CpGs tested are presented in Supplementary Tables S2–S4. Boxplots showing average DNAme β values in SSRI-exposed versus SSRI non-exposed cases for the differentially methylated CpGs are shown in Fig. 3.
Boxplots of differentially methylated CpGs by SSRI exposure. For all plots, points are colored by SSRI exposure (blue = SSRI non-exposed, dark yellow = SSRI-exposed), and boxplots indicate mean DNAme β value ± one standard deviation. (A) Boxplot of cg12900404, identified in the SSRI model, model A. (B) Boxplot of cg20877313, identified in the SSRI model, model A. (C) Boxplot of cg12655501, identified in the SSRIs in maternal depression model, model B. (D) Boxplot of cg26993610, identified in the SSRIs in maternal depression model, model B. (E) Boxplot of cg14340829, identified in the SSRIs in maternal depression model, model B.
A differentially methylated region (DMR) analysis was also conducted, to increase power for discovering DNAme-associations by grouping signatures from multiple neighboring CpG sites to reduce the number of statistical comparisons. DMR analysis was only conducted for the SSRI model to enable utilization of the entire cohort of cases and maximize statistical power.
M values were assessed for being part of DMRs for which average DNAme differed by SSRI exposure status using the DMRcate R package (FDR < 0.25, lambda = 1000, C = 2)61. Only one DMR was identified with an average DNAme β value that differed by SSRI exposure, this DMR comprised two CpGs 70 base pairs apart (cg06762403 and cg14921691) on chromosome 12, overlapping the DGKA gene. The average Δβ across the region was + 0.06, indicating higher DNAme in SSRI-exposed placentas, see Table 3 and Supplementary Fig. S4.
Few placental DNAme differences associated with SSRI exposure in sex-stratified models
As the effects of SSRI exposure might differ by sex, we next sought to assess whether unique SSRI-exposure DNAme associations arose in placentas of male versus female infants. Sex-stratified linear models were run in males (n = 33, 24% SSRI-exposed) and females (n = 31, 39% SSRI-exposed) to test for SSRI-exposure associated DNAme, adjusting for mean maternal Hamilton Depression Score, gestational age at birth, EPIC array row, and PlaNET ancestry. No CpGs were differentially methylated by SSRI exposure in males at any statistical threshold considered. In females, at an FDR < 0.25 and |Δβ| > 0.03, three CpGs (cg15849349, cg07481545, and cg03905236) were differentially methylated in association with SSRI exposure (Supplementary Fig. S5).
We previously identified 162 CpGs with robust sex differences in placental DNAme patterns that replicated in an independent dataset62. To investigate sex in another context, we tested whether these previously-ascertained sex-differentially-methylated CpGs were also differentially methylated by SSRI exposure status. All 162 replicated CpGs were considered, and were subjected to linear modelling in the discovery cohort for SSRI exposure, adjusting for mean maternal Hamilton Depression Score, gestational age at birth, EPIC array row, and PlaNET ancestry. No CpGs met significance at FDR < 0.05. At the low-confidence FDR < 0.25 threshold, three CpGs (cg22515303, cg27003571, cg26136722) had lower DNAme in SSRI-exposed placentas than non-exposed placentas, in addition to the previously identified sex effect at these loci (Supplementary Fig. S6).
No replication of DNAme signature associated with SSRI exposure in an independent dataset
We next intended to assess the reproducibility of the five CpGs with SSRI-associated differential DNAme identified in the SSRI model and SSRIs in maternal depression model (Table 3), and the two DMR CpGs. As the replication cohort was run on the Illumina 450K array, only four of these seven discovery cohort CpGs could be assessed for replication (cg14340829, cg20877313, cg06762403, cg14921691). A linear model was applied to the M values from these four CpGs in the replication cohort to test for differential DNAme associated with SSRI exposure, adjusting for infant sex, gestational age at birth, mode of delivery, and PlaNET ancestry. None of the four CpGs replicated differential methylation at a nominal p value < 0.05, see Supplementary Fig. S7.
Placental epigenetic age acceleration not associated with SSRI exposure or maternal depression
Epigenetic age is a useful dimension-reduction technique to analyze relationships between global DNAme patterns and a variety of health outcomes. Altered epigenetic age relative to chronological age (“epigenetic age acceleration”) has been associated with several negative health outcomes including overall greater burden of disease and higher rates of all-cause mortality63,64. Specific to depression, major depressive disorder is associated with increased epigenetic age acceleration in adult blood65. Umbilical cord blood epigenetic age deceleration was initially reported in maternal depression56, but interestingly this association has since been demonstrated to be largely related to the confounding signature of maternal SSRI treatment rather than depression itself57.
Using a recently published placental epigenetic clock55, we calculated epigenetic age acceleration in the discovery cohort. We then tested for associations between epigenetic age acceleration metrics and either SSRI exposure or mean maternal Hamilton Depression score using linear models. Neither epigenetic age acceleration nor intrinsic epigenetic age acceleration (cell-type independent) were significantly associated with either SSRI exposure or maternal depression, see Supplementary Fig. S8.
Replication of previously reported differential methylation candidates associated with SSRI-exposure and maternal depression in the discovery cohort
A previous study identified 16 CpGs with altered placental DNAme associated with maternal depression (treated as a categorical variable, where depression was maternal EPDS > 10) at FDR < 0.05 (no |Δβ| threshold)66. EPDS measurements were also collected for the discovery cohort cases in our study, and were significantly collinear with mean maternal Hamilton Depression scores across gestation, see Supplementary Fig. S9. To assess whether these previously-reported CpGs were also differentially methylated in association with maternal EPDS in the discovery cohort, we ran a linear model on the DNAme M values from these 16 CpGs, adjusting for infant sex, gestational age at birth, ancestry, and EPIC array row. One CpG (cg06670742) satisfied a nominal p value < 0.05 in our cohort, and had higher DNAme in cases with higher mean maternal EPDS scores (FDR < 0.05, Δβ = + 0.003). Thus, differential DNAme of this CpG did replicate in our study, however the effect size in our cohort was very small and did not exceed the technical threshold we set of |Δβ| > 0.03 (Fig. 4).
Assessment of literature candidates for SSRI and depression-associated DNAme in the discovery cohort. (A) Scatterplot showing the association between DNAme and maternal Edinburgh Postnatal Depression Score (EPDS) at cg06670742 which was previously found to be differentially methylated in association with maternal EPDS score in Tesfaye et al.37 and is differentially methylated by SSRI exposure at FDR < 0.05 in our discovery cohort. DNAme β value at this CpG is plotted along the Y axis, mean maternal EPDS score is plotted along the X axis and each point is a case; Δβ per unit increase in EPDS is Δβ = + 0.003. (B) Boxplot showing mean DNAme β value ± one standard deviation by SSRI exposure status at cg22159628 in ZNF575, previously found to be differentially methylated in cord blood with antidepressant exposure. Linear modelling p value = 0.67, points are colored by SSRI exposure (blue = SSRI non-exposed, dark yellow = SSRI-exposed), n.s. = not significant. (C) Boxplot showing mean DNAme β value ± one standard deviation by SSRI exposure status at cg00331486 in LOC284930 on chromosome 22, previously found to be differentially methylated in cord blood by SSRI exposure status.
Three previous studies investigated genome-wide DNAme alterations associated with SSRI treatment in a prenatal tissue (cord blood). An early study by Non et al. found no association between SSRI exposure and cord blood DNAme at any CpG loci67. Cardenas et al. reported one differentially methylated CpG in the gene body of ZNF575 associated with maternal antidepressant treatment in a cohort of cord blood samples68. In a different cohort, Kallak et al. reported 13 CpGs differentially methylated by SSRI exposure status in cord blood69. Though cord blood DNAme is very distinct from placenta, we sought to evaluate whether any of the 14 CpGs reported by Cardenas et al.68 or Kallak et al.69 were also differentially methylated by SSRI exposure status in the placenta. At the CpG reported by Cardenas et al. (cg22159528) we found no difference in DNAme by SSRI exposure (nominal p value > 0.05), see Fig. 4. At one of the 13 CpGs reported by Kallak et al., we found nominally significant (p < 0.05) differential DNAme associated with SSRI exposure status in the placenta in the same direction as the original cord blood association: higher DNAme in SSRI-exposed cases. This was cg00331486 in LOC284930 on chromosome 22, which encodes a MYC-interacting long noncoding RNA species69.
Discussion
In this study, we examined genome-wide placental DNAme patterns associated with SSRI exposure or maternal depression during gestation. Taken together, our results suggest the absence of a major impact of SSRI antidepressant exposure on the placental DNA methylome. Specifically, we found no differentially methylated CpGs associated with either SSRI exposure or maternal depression that met the standard significance threshold of FDR < 0.05. These results hold despite known alterations elicited by SSRIs to maternal gestational serotonin signalling and infant outcomes, suggesting that the effects of SSRI exposure on infant outcomes are not associated with a coincident DNAme signature in the placenta.
At more relaxed statistical thresholds, differential DNAme analysis highlighted five CpGs associated with SSRI exposure status. Three of the five differentially methylated CpGs overlapped the following genes, respectively: DOCK10 (CpG not present in replication cohort), GLS2 (CpG did not replicate), and TSPAN2 (CpG not present in replication cohort). The DOCK10 gene encodes the Dopamine Receptor Interacting Protein70, and was recently found to be one of the twelve most predictive mRNA biomarkers of depression in a gene expression study of adult whole blood71. These researchers identified that DOCK10 expression was higher in association with a more positive, less depressed mood71. DOCK10 is also a target of increased expression by the action of the antidepressant ketamine71. TSPAN2 encodes Tetraspanin-2, which mediates signal transduction in processes related to cellular growth and development72. Tspan2 has been reported to be upregulated in the rat hippocampus after chronic exposure to the SSRI fluoxetine73. We also identified one differentially methylated region in the 5′ untranslated region of DGKA, with higher DNAme in SSRI exposed placentas. DGKA encodes a diacylglycerol kinase that is involved in intracellular signalling74, and DGKA activity was recently reported to be inhibited by Ritanserin, a pharmaceutical serotonin receptor type 2 (5-HTR2) antagonist that is not currently in clinical use75.
Inherent to studying the impact of SSRI exposure are the concurrent, and potentially confounding, effects of maternal mood disturbances. As such, studies must be carefully designed to interrogate the effects of either SSRI treatment or depression exposure while accounting for the other factor. Only a limited placental DNAme signature has so far been associated with maternal mood disorders66, and to our knowledge no work has yet been conducted interrogating genome-wide placental DNAme signatures associated with SSRI exposure. Tesfaye et al.66 investigated placental DNAme in association with maternal depression, though SSRI treatment or antidepressant use was not reported on or investigated and could underlie some of these associations. Of the 16 CpGs they identified as differentially methylated by maternal depression status across six gestational time points, only one CpG in the EPS15L1 gene was also differentially methylated our study by mean maternal EPDS score across gestation (FDR < 0.05), and although the effect was in the same direction, the effect size (Δβ = + 0.003 with each unit increase in mean EPDS) was well below our biological |Δβ| threshold of > 0.03, and therefore may not be a biologically meaningful change in DNAme.
In non-placental tissue, Kallak et al.69 identified 13 CpGs that were differentially methylated in cord blood in association with SSRI exposure, one of which replicated at nominal significance in the same direction (higher DNAme in SSRI-exposed cases) in our cohort. Cardenas et al. also identified one CpG in the gene body of ZNF575 (cg22159528) with lower DNAme in antidepressant-exposed cord blood samples from Project Viva, which they replicated in an external cohort (Generation R Study)68. In our cohort, placental DNAme at this CpG was not associated with SSRI exposure. Although cord blood and placenta are both conceptus-derived tissues relevant to prenatal development and early life, they have unique origins (embryonic versus extraembryonic lineages, respectively) and thus distinct DNAme profiles are expected. Our finding that cord blood differential DNAme does not replicate at high rates in the placenta may suggest a cord-blood-specific DNAme signature at these CpGs in association with maternal SSRI treatment.
In sex-stratified analyses, three CpGs were differentially methylated by SSRI exposure in females: two in intergenic regions on chromosomes 4 and 6 respectively, and one in the 5’ untranslated region of the SH3GL3 gene. SH3GL3 encodes the Endophilin-A3 protein, which interacts selectively with the Huntingtin protein to promote the formation of polyglutamine-containing protein aggregates76. Promoter DNA methylation of the SH3GL3 gene was previously inversely correlated with its expression in human colon cancer cell lines versus matched double knockout cell lines for DNMT1 and DNMT3b77. Lower DNAme upstream of the promoter of the SH3GL3 gene in SSRI-exposed female placentas may indicate higher expression of this gene relative to the placentas from SSRI non-exposed females, which should be followed up in future work. In a second sex-focused analysis we tested whether previously reported sex-biased CpGs62 were differentially methylated by SSRI status, three CpGs met the low-confidence FDR < 0.25 threshold (two in the 5′ untranslated region of GTDC1, one in the gene body of C14orf132). Together, these analyses suggest that there may be a sex-biased signature of SSRI treatment on the placental DNA methylome or transcriptome, and future studies in this field should be designed to analyze sex as a variable of interest.
The major strength of our study lies in the curation of both SSRI treatment and maternal depression information per participant. This prospective design enabled us to investigate the potential confounding effects of these two variables in controlled contexts (i.e. studying SSRI exposure while adjusting for depression scores, and studying the effect of maternal depression in SSRI non-exposed samples). Additionally, in this study we measured genome-wide DNAme in an effort to reduce bias that arises from candidate gene or CpG pyrosequencing studies, and utilized the most modern Illumina EPIC array platform to capture the highest resolution data currently afforded by a DNAme microarray. Further strengths of our study include strict effect size and significance thresholds, the presence of an external replication cohort, the ability to account for covariates such as genetic ancestry and cell type proportions, and the ability to stratify analyses by sex.
Limitations of this work include SSRI exposure being defined as maternal treatment with any of six different SSRIs during gestation. It is possible that SSRI subtypes have different effects on placental DNAme, though this study was not able to undertake sub-analysis by SSRI type, and future work should consider this. The extent to which SSRI treatment improves maternal depression scores (i.e. remittance of symptoms) may also be associated with placental DNAme patterns and should be investigated in future longitudinal cohorts. Lastly, although sample size was relatively small, our cohort is among one of a handful of prospectively recruited cohorts that we are aware of with placental tissue collected alongside maternal depression and SSRI treatment information, including the BASIC and Generation R Studies78,79. Cord blood DNAme data exists for both of these cohorts and has previously been studied in association with maternal SSRI exposure68,69. Cohorts such as these, with extensive clinical records of maternal mood and medication use across gestation, are incredibly valuable resources to investigate how depression status and antidepressant medications may interact with fetal outcomes.
In conclusion, our work demonstrates that limited placental DNAme alterations are associated with maternal SSRI exposure; this work agrees with earlier findings of a similarly limited placental DNAme signature of maternal depression. Future work in larger cohorts will determine whether other molecular features are altered in association with maternal mood disorders or SSRI treatment. As larger cohorts with clinical characterization of maternal SSRI treatment and prenatal depressed mood become available, genetic sequence variation, gene expression patterns, DNA methylation alterations, and alterations in other epigenetic marks should all be considered, as well as their interactions.
Data availability
The raw and preprocessed EPIC array data and corresponding sample metadata supporting the conclusions of this article are available on the Gene Expression Omnibus (GEO), under the accession number GSE203396.
References
Andersson, L. et al. Point prevalence of psychiatric disorders during the second trimester of pregnancy: A population-based study. Am. J. Obstet. Gynecol. 189(1), 148–154 (2003).
Gorman, L. L. et al. Adaptation of the structured clinical interview for DSM-IV disorders for assessing depression in women during pregnancy and post-partum across countries and cultures. Br. J. Psychiatry Suppl. 46, s17-23 (2004).
Melville, J. L., Gavin, A., Guo, Y., Fan, M. Y. & Katon, W. J. Depressive disorders during pregnancy: Prevalence and risk factors in a large urban sample. Obstet. Gynecol. 116(5), 1064–1070 (2010).
Tran, H. & Robb, A. S. SSRI use during pregnancy. Semin. Perinatol. 39(7), 545–547 (2015).
Kim, D. R., O’Reardon, J. P. & Epperson, C. N. Guidelines for the management of depression during pregnancy. Curr. Psychiatry Rep. 12(4), 279–281 (2010).
Hanley, G. E. & Oberlander, T. F. The effect of perinatal exposures on the infant: Antidepressants and depression. Best Pract. Res. Clin. Obstet. Gynaecol. 28(1), 37–48 (2014).
Hanley, G. E., Brain, U. & Oberlander, T. F. Prenatal exposure to serotonin reuptake inhibitor antidepressants and childhood behavior. Pediatr. Res. 78(2), 174–180 (2015).
Goodman, S. H. & Halperin, M. S. Perinatal depression as an early stress: Risk for the development of psychopathology in children. In The Oxford Handbook of Stress and Mental Health (Oxford Library of Psychology) 287–312 (Oxford University Press, 2020).
Talge, N. M., Neal, C. & Glover, V. Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health. Antenatal maternal stress and long-term effects on child neurodevelopment: How and why?. J. Child Psychol. Psychiatry. 48(3–4), 245–261 (2007).
Hutchison, S. M., Mâsse, L. C., Pawluski, J. L. & Oberlander, T. F. Perinatal selective serotonin reuptake inhibitor (SSRI) and other antidepressant exposure effects on anxiety and depressive behaviors in offspring: A review of findings in humans and rodent models. Reprod. Toxicol. Elmsford N. 99, 80–95 (2021).
Oberlander, T., Gingrich, J. & Ansorge, M. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: Molecular to clinical evidence. Clin. Pharmacol. Ther. 86(6), 672–677 (2009).
Rurak, D. et al. Third trimester fetal heart rate and doppler middle cerebral artery blood flow velocity characteristics during prenatal selective serotonin reuptake inhibitor exposure. Pediatr. Res. 70(1), 96–101 (2011).
Rotem-Kohavi, N. & Oberlander, T. F. Variations in neurodevelopmental outcomes in children with prenatal SSRI antidepressant exposure. Birth Defects Res. 109(12), 909–923 (2017).
Levy, M. et al. Maternal use of selective serotonin reuptake inhibitors (SSRI) during pregnancy—neonatal outcomes in correlation with placental histopathology. J. Perinatol. 40(7), 1017–1024 (2020).
Clabault, H. et al. Effects of selective serotonin-reuptake inhibitors (SSRIs) on human villous trophoblasts syncytialization. Toxicol. Appl. Pharmacol. 349, 8–20 (2018).
Clabault, H., Cohen, M., Vaillancourt, C. & Sanderson, J. T. Effects of selective serotonin-reuptake inhibitors (SSRIs) in JEG-3 and HIPEC cell models of the extravillous trophoblast. Placenta 72–73, 62–73 (2018).
Thibeault, A. A. H., de Los Santos, Y. L., Doucet, N., Sanderson, J. T. & Vaillancourt, C. Serotonin and serotonin reuptake inhibitors alter placental aromatase. J. Steroid Biochem. Mol. Biol. 195, 105470 (2019).
Velasquez, J., Goeden, N. & Bonnin, A. Placental serotonin: Implications for the developmental effects of SSRIs and maternal depression. Front. Cell Neurosci. 7, 47 (2013).
Albert, P. R., Benkelfat, C. & Descarries, L. The neurobiology of depression—revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philos. Trans. R. Soc. B Biol. Sci. 367(1601), 2378–2381 (2012).
Moncrieff, J. et al. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry.. https://doi.org/10.1038/s41380-022-01661-0 (2022).
St-Pierre, J., Laurent, L., King, S. & Vaillancourt, C. Effects of prenatal maternal stress on serotonin and fetal development. Placenta 48(Suppl 1), S66-71 (2016).
Bonnin, A. et al. A transient placental source of serotonin for the fetal forebrain. Nature 472(7343), 347–350 (2011).
Laurent, L. et al. Human placenta expresses both peripheral and neuronal isoform of tryptophan hydroxylase. Biochimie 140, 159–165 (2017).
Karahoda, R. et al. Dynamics of tryptophan metabolic pathways in human placenta and placental-derived cells: Effect of gestation age and trophoblast differentiation. Front. Cell Dev. Biol. 8, 574034 (2020).
Karahoda, R. et al. Prenatal inflammation as a link between placental expression signature of tryptophan metabolism and preterm birth. Hum. Mol. Genet. 30(22), 2053–2067 (2021).
Campbell, K. S. J. et al. Maternal serotonin reuptake inhibitor antidepressants have acute effects on fetal heart rate variability in late gestation. Front. Psychiatry. 12, 680177 (2021).
Laurent, L. et al. In utero exposure to venlafaxine, a serotonin-norepinephrine reuptake inhibitor, increases cardiac anomalies and alters placental and heart serotonin signaling in the rat. Birth Defects Res. A Clin. Mol. Teratol. 106(12), 1044–1055 (2016).
Dhar, G. A., Saha, S., Mitra, P. & Nag, C. R. DNA methylation and regulation of gene expression: Guardian of our health. Nucleus 64(3), 259–270 (2021).
Martin, E. M. & Fry, R. C. Environmental influences on the epigenome: Exposure-associated DNA methylation in human populations. Annu. Rev. Public Health. 39, 309–333 (2018).
Chatterjee, S., Ouidir, M. & Tekola-Ayele, F. Genetic and in utero environmental contributions to DNA methylation variation in placenta. Hum. Mol. Genet. 30(21), 1968–1976 (2021).
Vlahos, A., Mansell, T., Saffery, R. & Novakovic, B. Human placental methylome in the interplay of adverse placental health, environmental exposure, and pregnancy outcome. PLoS Genet. 15(8), e1008236 (2019).
Burton, G. J. & Fowden, A. L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. B Biol. Sci. 370(1663), 20140066 (2015).
Pemathilaka, R. L., Reynolds, D. E. & Hashemi, N. N. Drug transport across the human placenta: Review of placenta-on-a-chip and previous approaches. Interface Focus. 9(5), 20190031 (2019).
Ewing, G., Tatarchuk, Y., Appleby, D. & Kim, D. Placental transfer of antidepressant medications: Implications for postnatal adaptation syndrome. Clin. Pharmacokinet. 54(4), 359–370 (2015).
Clifton, V. L. Review: Sex and the human placenta: Mediating differential strategies of fetal growth and survival. Placenta 31, S33–S39 (2010).
Bale, T. L. Sex differences in prenatal epigenetic programing of stress pathways. Stress. 14(4), 348–356 (2011).
Bale, T. L. The placenta and neurodevelopment: Sex differences in prenatal vulnerability. Dialogues Clin. Neurosci. 18(4), 459–464 (2016).
Gobinath, A. R., Workman, J. L., Chow, C., Lieblich, S. E. & Galea, L. A. M. Sex-dependent effects of maternal corticosterone and SSRI treatment on hippocampal neurogenesis across development. Biol. Sex Differ. 8, 20 (2017).
Sutherland, S. & Brunwasser, S. M. Sex differences in vulnerability to prenatal stress: A review of the recent literature. Curr. Psychiatry Rep. https://doi.org/10.1007/s11920-018-0961-4 (2018).
Campbell, K. S. J. et al. Prenatal antidepressant exposure and sex differences in neonatal corpus callosum microstructure. Dev. Psychobiol. 63(6), e22125 (2021).
Rohan, K. J. et al. A protocol for the Hamilton rating scale for depression: Item scoring rules, rater training, and outcome accuracy with data on its application in a clinical trial. J. Affect. Disord. 200, 111–118 (2016).
Murray, D. & Cox, J. L. Screening for depression during pregnancy with the Edinburgh depression scale (EDDS). J. Reprod. Infant Psychol. 8(2), 99–107 (1990).
Blair, J. D. et al. Widespread DNA hypomethylation at gene enhancer regions in placentas associated with early-onset pre-eclampsia. Mol. Hum. Reprod. 19(10), 697–708 (2013).
Paquette, A. G. et al. Regions of variable DNA methylation in human placenta associated with newborn neurobehavior. Epigenetics 11(8), 603–613 (2016).
Kramer, M. S. et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 108(2), E35 (2001).
Yuan, V. et al. Accurate ethnicity prediction from placental DNA methylation data. Epigenet. Chromatin. 12(1), 51 (2019).
Zhou, W., Laird, P. W. & Shen, H. Comprehensive characterization, annotation and innovative use of Infinium DNA methylation BeadChip probes. Nucleic Acids Res. 45(4), e22 (2017).
Edgar, R. D., Jones, M. J., Robinson, W. P. & Kobor, M. S. An empirically driven data reduction method on the human 450K methylation array to remove tissue specific non-variable CpGs. Clin. Epigenet. 9(1), 11 (2017).
Dieckmann, L. et al. Reference-based versus reference-free cell type estimation in DNA methylation studies using human placental tissue. in Review (2021) https://www.researchsquare.com/article/rs-848651/v1 (Accessed 13 Dec 2021).
Pidsley, R. et al. A data-driven approach to preprocessing Illumina 450K methylation array data. BMC Genomics 14, 293 (2013).
Leek, J. T., Johnson, W. E., Parker, H. S., Jaffe, A. E. & Storey, J. D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28(6), 882–883 (2012).
Nygaard, V., Rødland, E. A. & Hovig, E. Methods that remove batch effects while retaining group differences may lead to exaggerated confidence in downstream analyses. Biostatistics 17(1), 29–39 (2016).
Konwar, C., Del Gobbo, G., Yuan, V. & Robinson, W. P. Considerations when processing and interpreting genomics data of the placenta. Placenta 84, 57–62 (2019).
Yuan, V. et al. Cell-specific characterization of the placental methylome. BMC Genomics 22(1), 6 (2021).
Lee, Y. et al. Placental epigenetic clocks: Estimating gestational age using placental DNA methylation levels. Aging 11(12), 4238–4253 (2019).
Suarez, A. et al. The epigenetic clock at birth: Associations with maternal antenatal depression and child psychiatric problems. J. Am. Acad. Child Adolesc. Psychiatry. 57(5), 321-328.e2 (2018).
McKenna, B. G. et al. Maternal prenatal depression and epigenetic age deceleration: Testing potentially confounding effects of prenatal stress and SSRI use. Epigenetics 16(3), 327–337 (2021).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43(7), e47–e47 (2015).
Bass, A. et al. biobroom: Turn Bioconductor objects into tidy data frames. https://github.com/StoreyLab/biobroom.
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57(1), 289–300 (1995).
Peters, T. J. et al. De novo identification of differentially methylated regions in the human genome. Epigenet. Chromatin 8, 16 (2015).
Inkster, A. M. et al. A cross-cohort analysis of autosomal DNA methylation sex differences in the term placenta. Biol. Sex Differ. 12(1), 38 (2021).
Gale, C. R., Marioni, R. E., Harris, S. E., Starr, J. M. & Deary, I. J. DNA methylation and the epigenetic clock in relation to physical frailty in older people: The Lothian Birth Cohort 1936. Clin. Epigenet. 10(1), 101 (2018).
Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 14(10), R115 (2013).
Han, L. K. M. et al. Epigenetic aging in major depressive disorder. Am. J. Psychiatry. 175(8), 774–782 (2018).
Tesfaye, M., Chatterjee, S., Zeng, X., Joseph, P. & Tekola-Ayele, F. Impact of depression and stress on placental DNA methylation in ethnically diverse pregnant women. Epigenomics 13(18), 1485–1496 (2021).
Non, A. L., Binder, A. M., Kubzansky, L. D. & Michels, K. B. Genome-wide DNA methylation in neonates exposed to maternal depression, anxiety, or SSRI medication during pregnancy. Epigenetics 9(7), 964–972 (2014).
Cardenas, A. et al. Prenatal maternal antidepressants, anxiety, and depression and offspring DNA methylation: Epigenome-wide associations at birth and persistence into early childhood. Clin. Epigenet. 11(1), 56 (2019).
Kallak, T. K. et al. DNA methylation in cord blood in association with prenatal depressive symptoms. Clin. Epigenet. 13(1), 1–14 (2021).
DOCK10. (National Library of Medicine (US), National Center for Biotechnology Information, 2004) https://www.ncbi.nlm.nih.gov/gene/55619 (Accessed 25 Mar 2022).
Le-Niculescu, H. et al. Precision medicine for mood disorders: Objective assessment, risk prediction, pharmacogenomics, and repurposed drugs. Mol. Psychiatry. 26(7), 2776–2804 (2021).
TSPAN2. (National Library of Medicine (US), National Center for Biotechnology Information, 2004) https://www.ncbi.nlm.nih.gov/gene/10100 (Accessed 25 Mar 2022).
Kroeze, Y. et al. Long-term consequences of chronic fluoxetine exposure on the expression of myelination-related genes in the rat hippocampus. Transl. Psychiatry. 5(9), e642–e642 (2015).
DGKA. (National Library of Medicine (US), National Center for Biotechnology Information, 2004) https://www.ncbi.nlm.nih.gov/gene/1606 (Accessed 25 Mar 2022).
Boroda, S., Niccum, M., Raje, V., Purow, B. W. & Harris, T. E. Dual activities of ritanserin and R59022 as DGKα inhibitors and serotonin receptor antagonists. Biochem. Pharmacol. 123, 29–39 (2017).
Sittler, A. et al. SH3GL3 associates with the Huntingtin exon 1 protein and promotes the formation of polygln-containing protein aggregates. Mol. Cell. 2(4), 427–436 (1998).
Kang, J. Y. et al. Identification of long-range epigenetic silencing on chromosome 15q25 and its clinical implication in gastric cancer. Am. J. Pathol. 185(3), 666–678 (2015).
Axfors, C. et al. Cohort profile: The Biology, Affect, Stress, Imaging and Cognition (BASIC) study on perinatal depression in a population-based Swedish cohort. BMJ Open 9(10), e031514 (2019).
Jaddoe, V. W. V. et al. The generation R study: Design and cohort profile. Eur. J. Epidemiol. 21(6), 475–484 (2006).
Acknowledgements
We acknowledge all cohort owners and collaborators on the “Using ‘omics to build an atlas of placental development and function across pregnancy” (NIH Project). Thank you to Dr. Michael S. Kobor and the BCCHRI Microarray Core Facilities for support with Illumina EPIC DNAme runs. We thank Robinson lab members Ruby Jiang, Giulia F. Del Gobbo, and Desmond Hui for their work in sample processing, quality control, and testing, thank you to Victor Yuan and Ziqi Liu for pilot data processing for cases from this cohort. Finally, we sincerely thank the pregnant individuals and families that contributed placental samples to this study.
Funding
This work was supported by a grant from the National Institutes of Health (1R01HD089713-01). Collection of the Discovery Cohort was funded by a Canadian Institutes of Health Research grant (MOP-5783). WPR receives salary support through an investigatorship award from the BC Children’s Hospital Research Institute. AMI is funded by a Frederick Banting and Charles Best Canada Doctoral Scholarship from the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Contributions
A.M.I. conducted the analyses and wrote the manuscript, and contributed to sample processing, quality control, and testing. C.K. conducted a preliminary analysis of the effect of SSRI exposure on an earlier version of this data, and contributed to sample processing, quality control, and testing. M.S.P. is the NIH project manager, curated and organized clinical data, contributed to sample processing, quality control, and testing, and manuscript editing. U.B. conducted clinical data collection. A.K. processed and normalized the discovery cohort EPIC array data. E.M.P. and J.M.S. were involved in data management, sample metadata collation and establishment of the RedCap database, and participated in randomization. É.P.C. oversaw project administration and data curation of this cohort. A.B. performed data curation for the RICHS cohort cases, C.J.M. is cohort owner of the RICHS data and organized data curation for this set of cases. C.V. contributed to the conceptualization and design of this project, and also contributed to funding acquisition. T.F.O. and W.P.R. were responsible for project administration, funding acquisition, and supervision of the SSRI cohort recruitment and the DNAme data generation aims of the project, respectively, and were major contributors in writing and revising the manuscript. T.O. designed the original study. A.M.I., C.K., M.S.P., U.B., A.K., E.M.P., J.M.S., É.P.C., C.V., T.F.O., and W.P.R. all participated in experimental design. All authors read, revised, and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Inkster, A.M., Konwar, C., Peñaherrera, M.S. et al. Profiling placental DNA methylation associated with maternal SSRI treatment during pregnancy. Sci Rep 12, 22576 (2022). https://doi.org/10.1038/s41598-022-26071-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26071-8
This article is cited by
-
Effects of prenatal exposure to (es)citalopram and maternal depression during pregnancy on DNA methylation and child neurodevelopment
Translational Psychiatry (2023)
-
Epigenome-wide association studies of prenatal maternal mental health and infant epigenetic profiles: a systematic review
Translational Psychiatry (2023)
-
The application of epiphenotyping approaches to DNA methylation array studies of the human placenta
Epigenetics & Chromatin (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.